Abstract
Haptoglobin (HP) is the major scavenger of cell-free hemoglobin in circulation. When haptoglobin is depleted, cell-free hemoglobin can lead to organ damage through direct oxidative injury, upregulation of inflammatory pathways, and depletion of nitric oxide. A common polymorphism in HP gives rise to structurally and functionally distinct phenotypes: HP 1-1, HP 2-1, and HP 2-2. In people of African descent, the HP 1-1 and 2-1 phenotypes have been associated with higher serum HP concentrations compared to the HP 2-2 phenotype (PMID 10807979). In hemolytic diseases, such as sickle cell disease (SCD), the consequence of the HP polymorphism is unclear but could influence cell-free hemoglobin processing and susceptibility to organ damage.
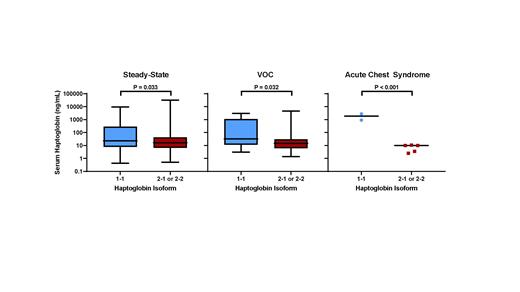
We first investigated whether the HP isoforms are associated with HP concentrations at steady-state. Between 10/2009 and 6/2018, we recruited 431 SCD patients into a longitudinal registry at our institution during a routine clinic visit, which we defined as steady-state. Baseline clinical and laboratory data were collected at the time of enrolment. The median age of the cohort was 32 (interquartile range [IQR], 24 - 43) years, 43% were male, 76% were SCD SS or Sβ 0-thalassemia genotype, and 46% were on hydroxyurea at the time of enrolment. Genotyping of the HP polymorphisms by PCR demonstrated the HP 1-1 isoform in 30% (n = 129), the HP 2-1 isoform in 47% (n = 203), and the HP 2-2 isoform in 23% (n = 99) of the SCD cohort. Serum samples were available in 244 of the 431 SCD patients at steady-state. Serum concentrations of HP, measured by ELISA, were higher in those with the HP 1-1 isoform compared to the HP 2-1 or 2-2 isoform (Figure). In a linear regression model, independent variables associated with steady-state serum HP concentration included SCD SS or Sβ 0-thalassemia genotype (β -1.8; 95% CI: -2.5 to -1.0; P < 0.001), LDH (natural log β -0.9; 95% CI: -0.2 to -1.6; P = 0.018), hydroxyurea use (β 0.6; 95% CI: 0.1 to 1.1; P = 0.02), and HP 1-1 isoform (β 0.5, 95% CI: 0 to 1.1; P = 0.049), adjusting for age and sex.
Between 9/2020 and 5/2021, we collected an additional 38 serum samples from SCD patients during a hospitalization for vaso-occlusive crisis (VOC) and serum samples from 7 SCD patients during a hospitalization for acute chest syndrome. The HP 1-1 isoform was associated with higher serum HP concentrations during VOC and during acute chest syndrome compared to the HP 2-1 or 2-2 isoforms (Figure). Interestingly, the serum HP trended higher from steady-state to VOC and to acute chest syndrome in SCD patients with the HP 1-1 isoform (β +1.1, P = 0.085) but trended lower in those with the HP 2-1 or 2-2 isoform (β -0.5, P = 0.099).
Next we tested whether the HP 1-1 isoform is protective against multi-organ failure during hospitalizations for acute chest syndrome. For the longitudinal analysis, we focused on SCD patients with > 6 months of follow up at our institution (n = 391). A multiorgan failure event was defined as organ dysfunction involving at least 2 of the following organ systems: lung, kidney, liver, or central nervous system (PMID 8109600). With a median follow up of 6.8 (IQR, 3.5 - 8.9) years, we observed an acute chest syndrome episode complicated by multiorgan failure in 14% (53/391) of SCD patients. A significantly lower proportion of SCD patients with the HP 1-1 isoform developed a multiorgan failure event versus those with the 2-1 or 2-2 isoforms (5.7% vs. 17.2%, respectively; P < 0.001). On logistic regression analysis, acute chest syndrome with multiorgan failure was associated with the HP 1-1 isoform (OR 0.3, 95% CI: 0.1 to 0.6; P = 0.002) and baseline LDH (natural log OR 2.7, 95% CI: 1.2 - 6.3; P = 0.02), after adjusting for age, sex, SCD genotype and hydroxyurea use.
In conclusion, we demonstrate that the HP 1-1 isoform is independently associated with higher serum HP concentrations at steady-state as well as during hospitalizations for VOC or acute chest syndrome compared to the HP 2-1 or 2-2 isoforms. Furthermore, the HP 1-1 isoform is protective against developing the devastating multiorgan failure syndrome during acute chest syndrome episodes. Our findings highlight the clinical significance of the HP isoforms and cell-free hemoglobin scavenging in SCD. Future studies assessing HP induction and scavenging function based on HP isoform may help elucidate high-risk patients for aggressive measures, such as rapid initiation of exchange transfusion therapy or HP replacement therapy.
Gordeuk: Modus Therapeutics: Consultancy; Novartis: Research Funding; Incyte: Research Funding; Emmaus: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; CSL Behring: Consultancy. Saraf: Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal